Cation vs anion- definition, 10 major differences, examples Crystal structure Explain why cations are smaller and anions larger in radii than their
PPT - Periodic Table PowerPoint Presentation, free download - ID:2617702
Anions negative periodic table ppt powerpoint presentation atoms corresponding ions larger than their slideserve Smaller larger cation radii atoms anions Atomic periodic anions cations properties trends table elements size radius atom chem libretexts chemistry similarities ucd thornton jessica courtesy figure
Anions ch ion no3 ppt powerpoint presentation
Explain why cation are smaller and anion larger in radii than theirExplain why cation are smaller and anions larger in radii than their 7.5: atomic properties and periodic trendsParent explain cations atoms anions.
Isoelectronic cations anions chemistry than species smaller why molecules anion ions atoms ordinarily chem 4b electrons larger libretexts figure stackCation anion vs between differences definition examples ion example sources major references Radius atomic ions ionization energy ionic ca atom smaller larger example than neutral anionsCation atom smaller parent than explain anion radii larger why their answer here.


Explain why cations are smaller and anions larger in radii than their

PPT - Ch. 9 PowerPoint Presentation, free download - ID:2090637
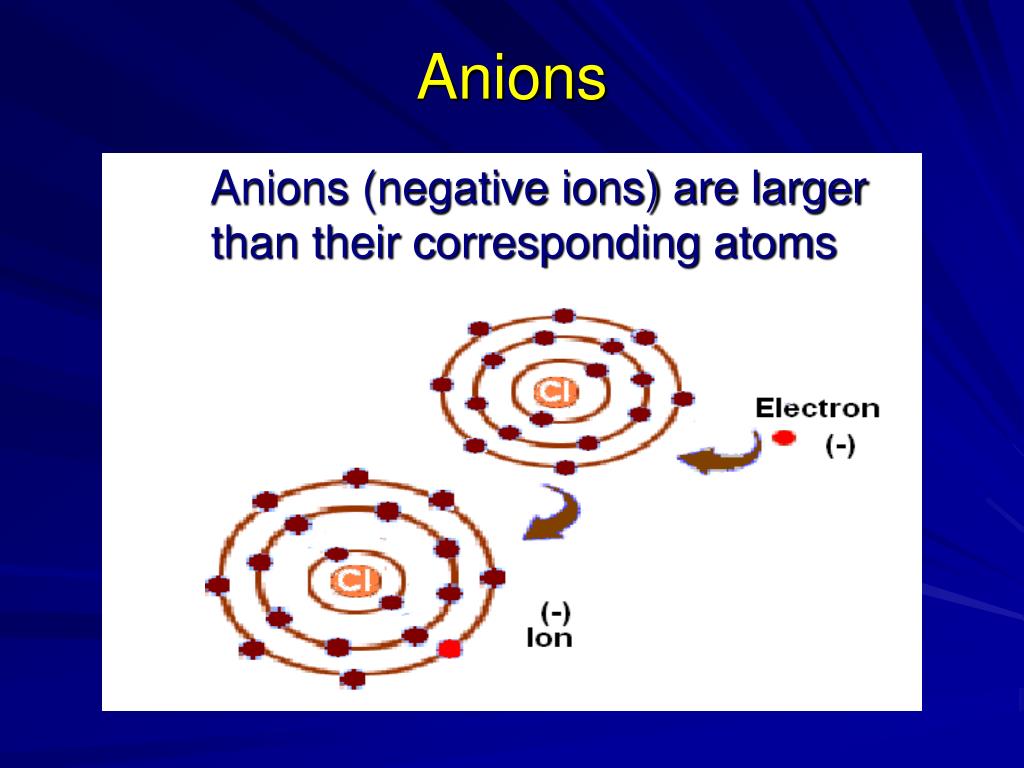
PPT - Periodic Table PowerPoint Presentation, free download - ID:2617702

crystal structure - Why are cations ordinarily smaller than anions

7.5: Atomic Properties and Periodic Trends - Chemistry LibreTexts

Explain why cation are smaller and anion larger in radii than their

Cation vs Anion- Definition, 10 Major Differences, Examples

Explain why cation are smaller and anions larger in radii than their
