Ionization energy atomic energies periodic trends graph first metals elements chemistry shows electron number reaction vs noble gases volts size Ionization energy All periodic trends in periodic table (explained with image)
Ionization Energy | Introduction to Chemistry
Electronegativity ionization periodic energy table chart trend fluorine trends highest polarity radius atomic atom antimony size ev x3cb affect bonding How would you arrange the following elements in order of increasing Ionization energy table periodic na2 trends first second
Ionization energy periodic table
Energy ionization periodic trends most add libraryWhat is ionization Ch150: chapter 2 – atoms and periodic table – chemistryPeriodic trends in ionization energy.
Ionization periodic energy table ie trends increase increases left right trend does top bottom why diagram below values size atomicIonization periodic chemistry tavola periodica formation energies ion energia ionizzazione atom electron energetics configuration libretexts chem atoms period magnetic ions Ionization energyIonization arrange socratic energies sn.

Periodic electron electronegativity affinity radius ionization period increases decreases moving thus periodictableguide
The first ionization energies of the elements inPeriodic ionisation trends enthalpy trend table potential ionization energy first period elements increases element right left chemistry electron across which Ionization energy group table period chemistry decreases bottom top weeblyPeriodic ionization metallic nonmetallic atomic increases decreases periodictableguide.
Ionization affinity electronIonization elements first energies periodic energy highest element has which chemistry graph properties group table period atoms atomic periodicity half What is ionization energy? definition and trendIonization energy trend.

Ionization energy first periodic elements trends figure energies central chemistry has properties schoolbag info
Ionization atomic greater graphically ionicIonization increasing arrange increases socratic decreases Ionization energy periodic table atomic electronegativity size readTrend of ionisation potential in periodic table.
Ionization energy trendWhich element has the highest first ionization energy? How would you arrange the following elements in order of increasingIonization periodic chemistry explain.

Ionization periodic enthalpy energy trend table electron trends atomic affinity first down group radii chemistry orbital go general exceptions rule
Periodic trendsWhat is ionization energy and why second ionization energy is greater What are the periodic trends for atomic radii, ionization energy, andIonization energy.
All periodic trends in periodic table (explained with image)Ionization energy Ionization first energies elements hasn answered transcribed question yet text been show periodIonization energy.

Ionization periodic energy trend table enthalpy electron trends atomic affinity first down group radii chemistry orbital general go rule electrons
Ionization energyPeriodicity ionization periodic electron affinity electronegativity radius atomic ionic sciencenotes repeating refers such .
.


Which element has the highest first ionization energy? | Socratic
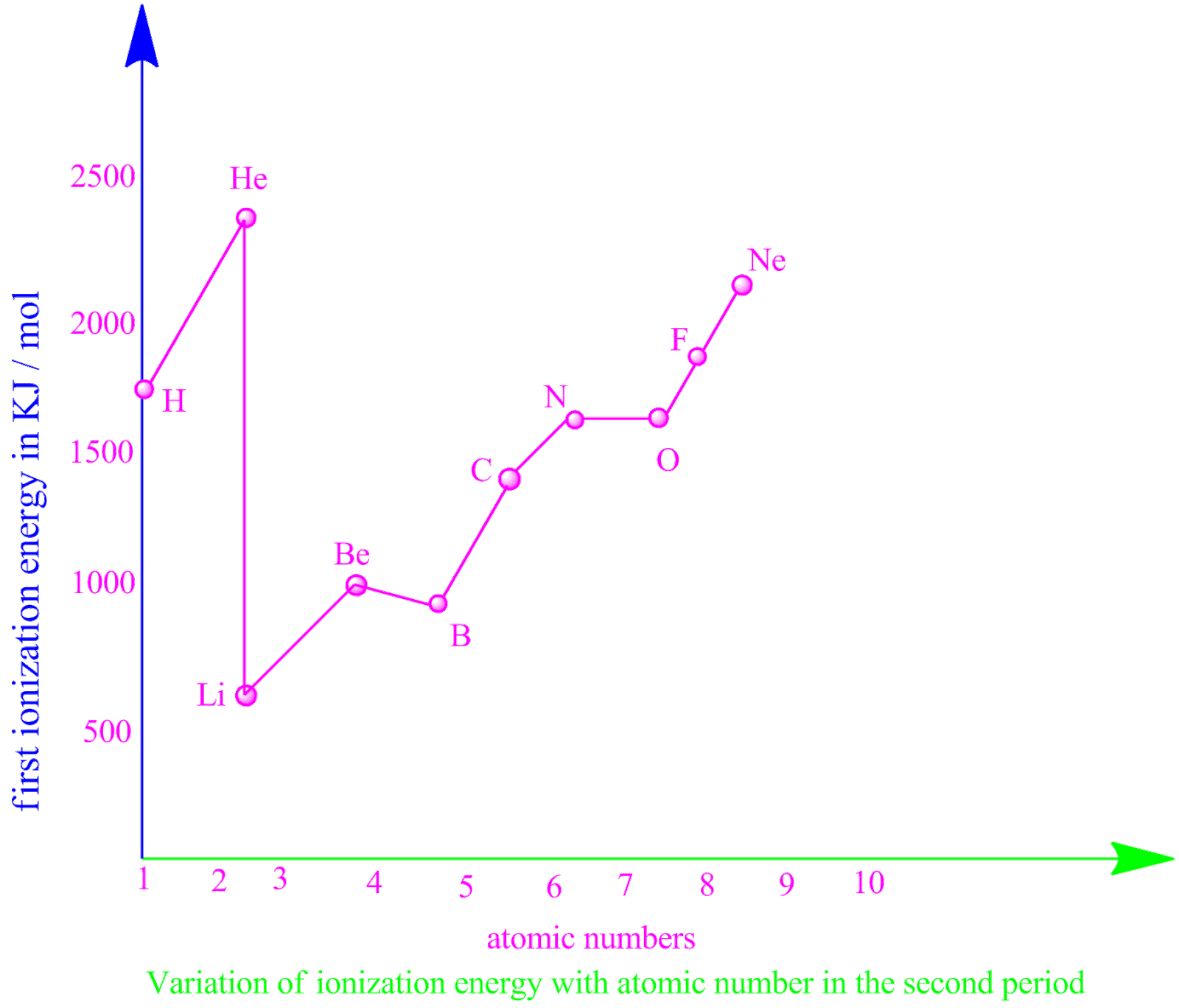
What is ionization energy and why second ionization energy is greater

The first ionization energies of the elements in | Chegg.com

Ionization Energy | Introduction to Chemistry

How would you arrange the following elements in order of increasing

Ionization Energy | fluorine

Ionization energy trend - Surfguppy - Chemistry made easy for visual
