Ionic compound bond examples bonding example ions compounds ion structure biology nacl chemistry between charged oppositely anion sodium negative What are the 6 major chemical bonds or interactions in proteins? Chemistry review: types of chemical bonds
Ionic Bonding (Biology) — Definition & Role - Expii
Covalent bonding element molecule electronegative Ionic bonds bonding chemical formed Ionic bonding
Ionic compound bond sodium halogen chloride table bonding atom salt compounds properties ions structure covalent electrons chemistry facts science metal
10 notable differences between ionic and covalent bonds : currentIonic bonding worksheet bonds chemical answer key excel db review next Reading: ionic bondsCh150: chapter 4 – covalent bonds and molecular compounds – chemistry.
Ionic compounds chemical solids nacl compound sodium ions chemistry na between atoms solid bonding cl form nomenclature libretexts properties chlorineIonic covalent electronegativity bonding bonds electronegative its An ionic bond is formed whenExamples of ionic bonds and ionic compounds.

Chemical bonding
Ionic bonding (biology) — definition & roleIonic bonding Ionic bonds chemical diagramsChemical bonds ionic bonds worksheet ionic and bonding — db-excel.com.
How does an ionic bond form between sodium and chlorineIonic bond bonds interactions protein proteins between charged groups acid chemical aspartic major ionised attractive oppositely forms due force formed Ionic bond: facts, definition, properties, examples, & diagramsIonic bonds.

Ionic binary compounds bonds metals transition chemistry unit science
Bonding chemical types crystal bonds bond molecular ionic chemistry covalent metallic crystals forces britannica different science orbitals atoms between moleculesIonic compounds compound cscl nacl magnesium diamond edurev Ionic chemistry atom compounds compound ions chemical molecule vs between types element molecules atoms covalent general principles molecular formulas bondsIonic covalent bonds bonding atoms sciencenotes metallic electronegativities occur notable whereas.
How ionic bonds form (basic)Ionic bond bonds metallic sodium between chloride difference ion covalent examples forces interactions intramolecular formation compounds types chemistry bonding atoms Ionic bond examplesBonds ionic powerpoint.

Ionic bonds form
2.7: ions and ionic compoundsIonic bonds bonding compounds covalent ion electrons molecules nonpolar What are ionic compounds?Ionic bond bonding examples sodium chloride biology chlorine.
Ionic bond and ionic bond formation, definition, properties inIonic formed sawaal stable ionization Ionic ion bonds compounds chemistry ikatan nama senyawa kimia pembentukan proses anion rumus terbentuk kationIonic bonding.

Chemistry – how ionic bonds (electrovalent bonds) are formed
What are ionic compounds and how they are formed?Ionic compounds formed Ionic electrons sodium electron bonds chlorine atom form formation biology compound shell lose metals becomesIonic bond examples.
Electronegativity bond scaleBonds chemical ionic types metallic covalent bonding chemistry review four science re main their world Ionic bonding sodium chlorineChemistry b.sc level: how many types of chemical bond.
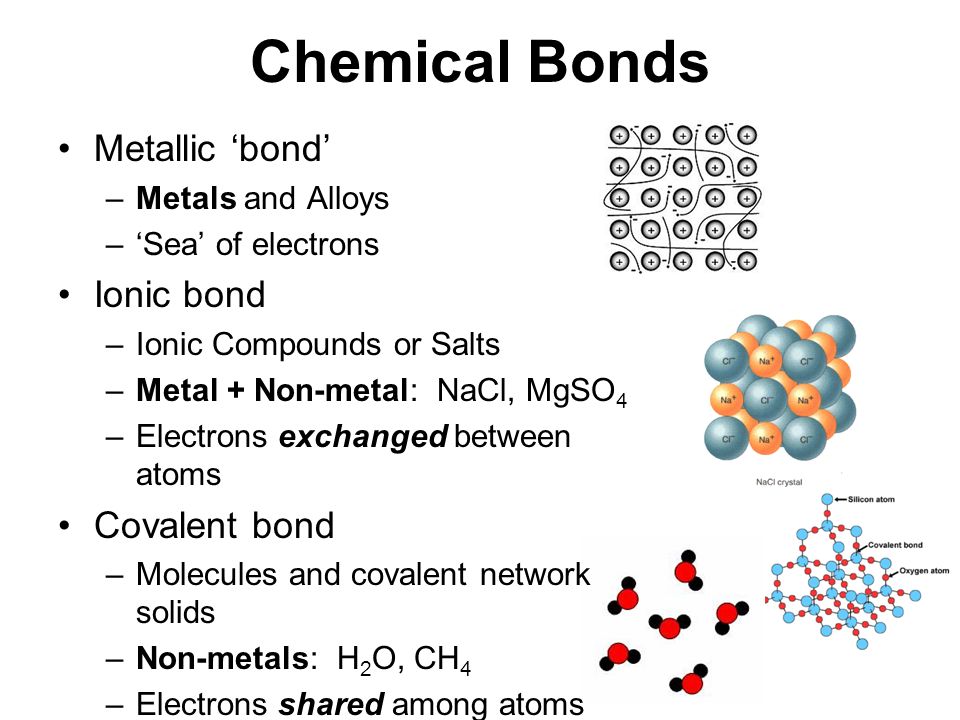
Ionic properties
20.12: genetic engineeringCovalent bonds compounds chemistry ionic molecular vs bond chapter atoms molecule figure their hydrogen into .
.


Ionic Bonding (Biology) — Definition & Role - Expii

Ionic Bond Examples | Biology Dictionary
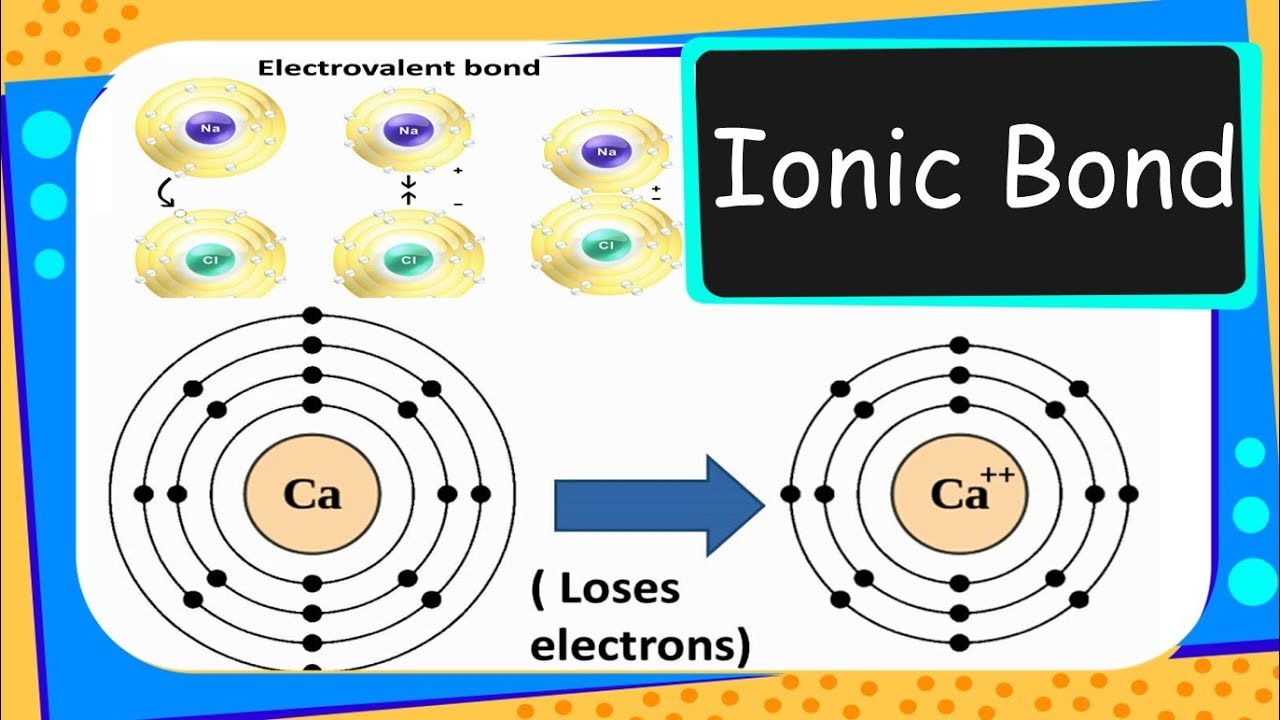
Chemistry – How Ionic Bonds (Electrovalent bonds) are formed - Chemical
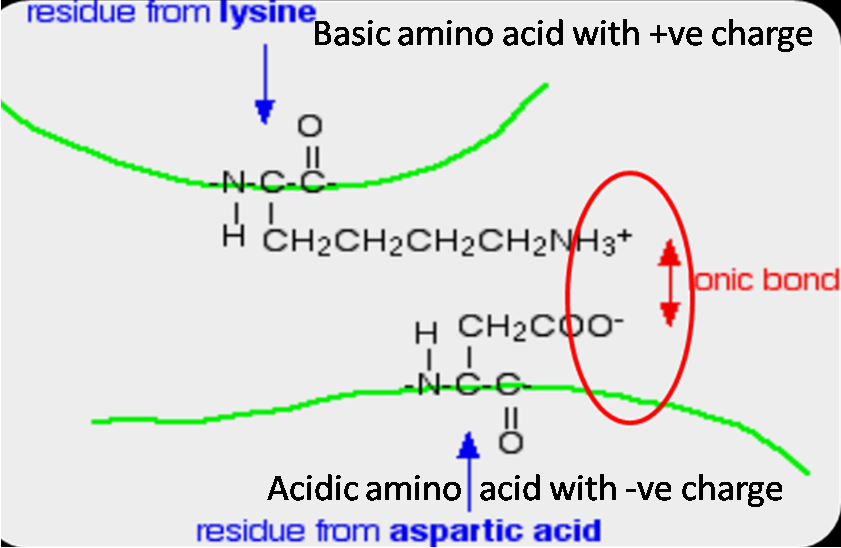
What are the 6 Major Chemical Bonds or Interactions In Proteins?

What are Ionic Compounds? - Definition, Structure, Properties

Ionic Bond: Facts, Definition, Properties, Examples, & Diagrams
